Researchers hope to begin world’s first human challenge trials at east London quarantine facility in January
Healthy volunteers in the UK could soon be deliberately infected with coronavirus in the world’s first human challenge trial to find out which Covid vaccines work.Government-funded studies, which it is believed will be announced next week, could begin in January in London, the Financial Times has reported. Volunteers exposed to Covid-19 would be closely monitored in strict quarantine for as long as a month.
Human challenge studies have a long and successful history, dating back to the end of the 18th century, when Edward Jenner inoculated an eight-year-old boy with cowpox virus and then exposed him to smallpox. They have also been used in the hunt for vaccines for typhoid, cholera and malaria. But some have qualms about exposing volunteers to a virus for which there is no cure.
The risks to young, fit and healthy people are low, however, and 2,000 UK volunteers have signed up to the 1Day Sooner movement, which has been campaigning and petitioning parliament to allow human challenge trials to begin. The movement has 37,000 willing participants worldwide.
Such trials could produce quick and robust answers about the effectiveness of some of the vaccines in advanced development. There are more than 300 putative vaccines, mostly in the lab, and nine now in final large-scale phase 3 trials. Finding out which ones can protect at least some people from infection would help decide where the effort and investment should go.
During the trial, volunteers will be injected with an experimental vaccine, before receiving a “challenge” dose of Sars-Cov-2 – the virus that causes Covid-19 – under controlled conditions around a month or so later.
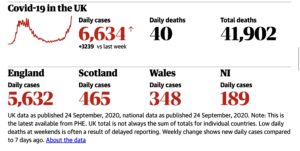 The FT reported that 1Day Sooner would launch a campaign this week with a petition to parliament asking for public funding of a biocontainment facility with capacity to quarantine 100 to 200 participants.
The FT reported that 1Day Sooner would launch a campaign this week with a petition to parliament asking for public funding of a biocontainment facility with capacity to quarantine 100 to 200 participants.
